Vortek® & Vortek® Hydro Ureteral Stents
Vortek® & Vortek® Hydro ureteral stents are the desirable go-to stents for drainage of the urinary tract and healing of the ureter. Vortek® ureteral stents sized 6-8Fr feature a stiff inner layer that aids in insertion over a guidewire and a soft outer layer that promotes patient comfort. The hydrophilic-coated Vortek® Hydro further aids in insertion by improving the glide between the stent and epithelial layer of the ureter.

Quick Links: Vortek® | Vortek® Hydro | Resources | Subscribe to emails | Ordering information | Important safety information

Vortek®
Double loop ureteral stents
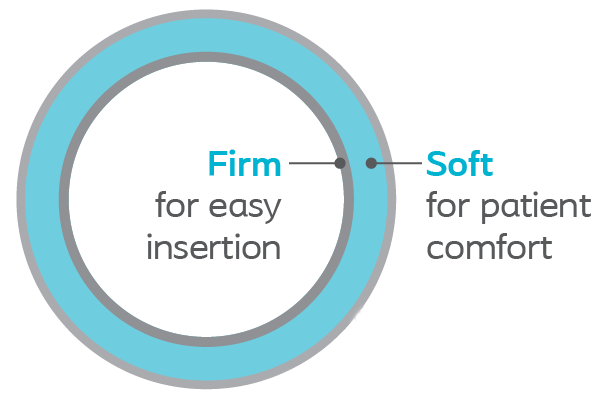
The material of choice to ease insertion through narrow ureteral paths.
Dual Durometer material on sizes 6-8Fr for easy insertion and placement while retaining great flexibility for patient comfort. Thermosensitive material is firm for advancement but softens at body temperature for increased patient comfort.
Indwelling time of up to 6 months
Kit composition:
|  |

Vortek® Hydro
Hydrophilic-coated double loop ureteral stent kit
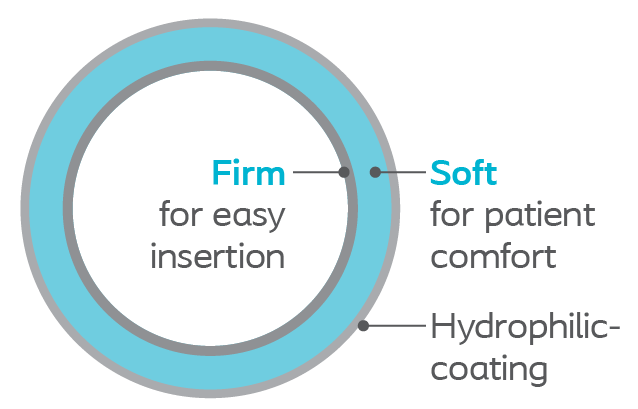
Improved glide and facilitated insertion with Hydrophilic-coating.
Dual durometer material for easy insertion and placement while retaining great flexibility for patient comfort. Thermosensitive material is firm for advancement but softens at body temperature for increased patient comfort.
Indwelling time of up to 6 months
Kit composition:
|  |
Subscribe for more from Coloplast Endourology
Sign up to receive information and support for Endourology Your Way. By opting into our emails, we can keep in touch with you on new products and updates.
"*" indicates required fields
Vortek® Ordering Information
| Diameter (Ch/Fr) | Length (cm) | With Seldinger Guidewire 0.035″ | Without Guidewire |
| 1 Each | 1 Each | ||
| Stent 4.8Ch/Fr + Pusher 6Ch/Fr 40 cm | 12 | ACB1C0 | ACBM50 |
| 16 | ACB1C1 | ACBM51 | |
| 20 | ACB1C2 | ACBM52 | |
| 22 | ACB1C7 | ACBM57 | |
| 24 | ACB1C3 | ACBM53 | |
| 26 | ACB1C4 | ACBM54 | |
| 28 | ACB1C5 | ACBM55 | |
| 30 | — | ACBM56 | |
| 6 | 20 | ACB162 | ACBM62 |
| 22 | ACB167 | ACBM67 | |
| 24 | ACB163 | ACBM63 | |
| 26 | ACB164 | ACBM64 | |
| 28 | ACB165 | ACBM65 | |
| 30 | ACB166 | ACBM66 | |
| 7 | 20 | ACB172 | ACBM72 |
| 22 | ACB177 | ACBM77 | |
| 24 | ACB173 | ACBM73 | |
| 26 | ACB174 | ACBM74 | |
| 28 | ACB175 | ACBM75 | |
| 30 | ACB176 | ACBM76 | |
| 8 | 24 | ACB183 | ACBM83 |
| 26 | ACB184 | ACBM84 | |
| 28 | ACB185 | ACBM85 | |
| 30 | ACB186 | ACBM86 |
Vortek® Hydro Ordering Information
| Diameter (Ch/Fr) | Length (cm) | With Seldinger Guidewire 0.035″ | With Orchestra® Guidewire 0.035″ | Without Guidewire |
| 1 Each | 1 Each | 1 Each | ||
| Stent 4.8Ch/Fr + Pusher 6Ch/Fr 40 cm | 22 | BCFA47 | BCFS47 | BCFD47 |
| 24 | BCFA43 | BCFS43 | BCFD43 | |
| 26 | BCFA44 | BCFS44 | BCFD44 | |
| 28 | BCFA45 | BCFS45 | BCFD45 | |
| 6 | 22 | BCFA67 | BCFS67 | BCFD67 |
| 24 | BCFA63 | BCFS63 | BCFD63 | |
| 26 | BCFA64 | BCFS64 | BCFD64 | |
| 28 | BCFA65 | BCFS65 | BCFD65 | |
| 30 | BCFA66 | BCFS66 | BCFD66 | |
| 7 | 22 | BCFA77 | BCFS77 | BCFD77 |
| 24 | BCFA73 | BCFS73 | BCFD73 | |
| 26 | BCFA74 | BCFS74 | BCFD74 | |
| 28 | BCFA75 | BCFS75 | BCFD75 | |
| 30 | BCFA76 | BCFS76 | BCFD76 |
Ordering information
For ordering information, please call 800-258-3476.