ReTrace® Access Sheath
Ready, Set, ReTrace
Putting choice in your hands

Quick Links: Product Detail | Product Resources | Ordering Information | Important Safety Information
Unlimited access
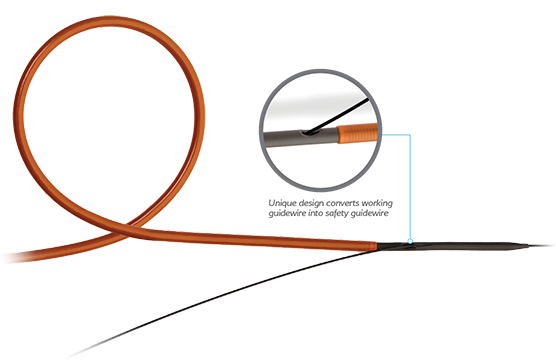
ReTrace ensures usability in both traditional and single wire methods with ease
- ReTrace secures access in one simple, repeatable step
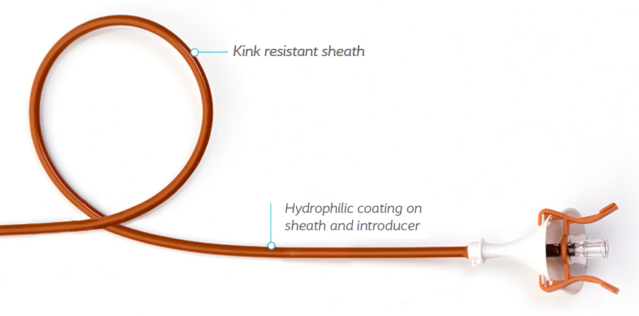
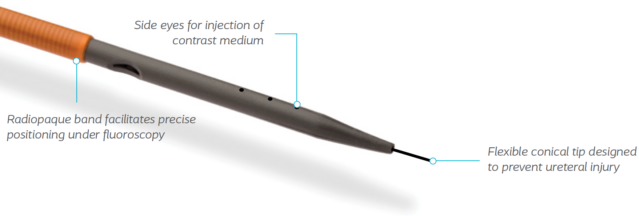
- Positioning and placement are easy with radiopaque introducer and ring
- A white proximal funnel allows visualization of urine and
irrigation colors - Reinforced structure ensures high kink-resistance
- Perform a retrograde pyelogram with or without a guidewire in place
Secure savings
With ReTrace, your procedure requires less tools and less time.
- The single wire insertion technique of ReTrace eliminates the need for a dual lumen catheter and a second wire
- ReTrace single wire placement minimizes the time and steps to perform the procedure compared to a standard access sheath

Complete comfort
ReTrace is designed to respect your patient’s anatomy and prevent injury
- Innovated to be more flexible than other access sheaths
- A flexible tip design was created to negotiate difficult portions of the ureter
- Hydrophilic coating facilitates placement in the procedure
*Device images are not drawn to scale and are for illustrative purposes only
-
Marketing Materials
Videos
Resources
Marketing Materials
ReTrace Product Brochure Send a SMS Share
Videos
ReTrace Product Animation Send a SMS Share
Clinical Evidence
First Clinical Evaluation of a New Innovative Ureteral Access Sheath (ReTrace) a European Study Send a SMS Share
Ordering information
For ordering information, please call 800-258-3476.
| Item | Description | Sales UOM | EA / Sales UOM |
| ASXL10 | 10-12 Fr, 28 cm length | Each | 1 EA |
| ACXL10 | 10-12 Fr, 35 cm length | Each | 1 EA |
| AXXL10 | 10-12 Fr, 45 cm length | Each | 1 EA |
| ALXL10 | 10-12 Fr, 55 cm length | Each | 1 EA |
| ASXL12 | 12-14 Fr, 28 cm length | Each | 1 EA |
| ACXL12 | 12-14 Fr, 35 cm length | Each | 1 EA |
| AXXL12 | 12-14 Fr, 45 cm length | Each | 1 EA |
| ALXL12 | 12-14 Fr, 55 cm length | Each | 1 EA |